- Joined
- Nov 17, 2011
- Messages
- 13,771
RightTherefore, should I perform the same discharge test on the cells down to a cut off voltage of 2.5V?
Mathematically incorrect3200mAh = 3.2A
3.2A x 0.2C = 0.64A
RightTherefore, should I perform the same discharge test on the cells down to a cut off voltage of 2.5V?
Mathematically incorrect3200mAh = 3.2A
3.2A x 0.2C = 0.64A
Right
Mathematically incorrect, but you get the gist
. 0.64 A is correct.

I think your question has been answered. You discharged to 3V, they discharged to 2.5V when rating the capacity. This would account for the difference.
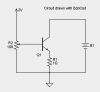
To answer your question though, you need a constant current sink to draw 620mA.
Here is a simple circuit:
View attachment 34752
The supply labeled 2V must be a stable reference voltage independent of the battery, since the battery voltage will be changing. You should get 620mA with something close to 1.2V on the base of Q1.
Bob
No, that is not what the conversion rate would mean. The 85% is for the conversion from the battery voltage to the regulated output.
Bob
